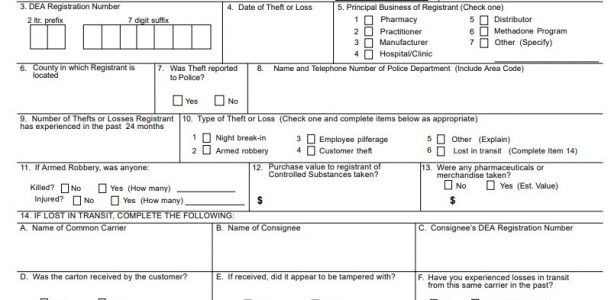
Registrants who notice discrepancies with controlled substances are required under federal regulations to notify the Field Division Office of the Administration in writing. The registrant must report a loss or theft of controlled substances in writing within one business day of discovering the theft or loss.
DEA Form 106 must be completed in its entirety and submitted to the Field division office in the registrant’s area. DEA Form 107 is required to report the theft or loss of listed chemicals. Note that only those that are registered and/or regulated with DEA to handle listed chemicals or controlled substances can complete and submit these forms.
For more information on the reporting required for the theft or loss of controlled substances under the Drug Enforcement Administration, DEA, 21 CFR, see the Diversion Control Division of the US Department of Justice, Drug Enforcement Administration.
C2R Global Manufacturing, Inc. Helps to Prevent Drug Diversion
Following compliant drug disposal methods is critical to meet DEA regulations and prevent drug diversion.C2R Global Manufacturing, Inc. offers a host of products which provide quick and easy drug disposal which meet DEA requirements for neutralizing medications with chemical digestion. We offer a wide selection of various containers to meet any facility needs from local pharmacies to nursing homes and hospitals. Incorporating proven products from Rx Destroyer™ with a comprehensive plan for managing pharmaceuticals and controlled substances helps to prevent diversion, environmental contamination and hefty fines for noncompliance.
Facilities should maintain a proper chain of custody for all controlled substances with witness logs to track the control, custody and disposition of all controlled substance medications maintained and healthcare settings. C2R Global Manufacturing, Inc. is dedicated to safe and effective pharmaceutical waste management and disposal, offering turnkey solutions with compliant consultations, waste haulers and mail back programs. Our customers have come to rely on our industry expertise and patented* products which quickly neutralize medications, preventing drug diversion.
C2R Global Manufacturing, Inc. is a leading provider of pharmaceutical waste disposal products along with education, training and implementation. We offer turnkey solutions for compliant pharmaceutical waste management and industry expertise. Contact us to learn how to implement safe, easy, and compliant pharmaceutical waste disposal of controlled substances.